

Sodium chloride/Molar mass How many units are present in a mole?Ī mole (mol) is the amount of a substance that contains 6.02 × 10 23 representative particles of that substance. Once you know the number of moles of CaO, you can determine the number of formula units by multiplying the number of moles by 6.022×1023. You need to determine the number of moles in 0.335 g CaO. How do you find the number of formula units in a mole?Įxplanation: There are 6.022×1023 in 1 mole of anything, including formula units. This would give you the mass of 1 mol of sodium chloride which would be about 58.443 g. If you had the compound sodium chloride (NaCl), you would add the mass of 1 mol of sodium and 1 mol of chlorine. 8 How many atoms are in a mole of carbon-12?.3 How many units are present in a mole?.2 How do you find the number of formula units in a mole?.“Interpreting and propagating the uncertainty of the standard atomic weights (IUPAC Technical Report)”. Possolo, Antonio van der Veen, Adriaan M.The International System of Units (SI) (8th ed.). International Bureau of Weights and Measures (2006).Oxford: Blackwell Scientific Publications. Compendium of Chemical Terminology (the “Gold Book”) (2nd ed.). Quantities, Units and Symbols in Physical Chemistry (2nd ed.). International Union of Pure and Applied Chemistry (1993).Molar mass finds value in mixtures, where molecular mass isn’t applicable. Another use of molar mass is finding average mass per mole of DNA or RNA, which contain varying numbers of different nucleotides. A more common scenario is calculating the molar mass of a sample of a polymer that contains differing numbers of monomer subunits. So, the molar mass of NaCl presumably is different in a sample from Earth versus one collected on Venus because of slight variations in isotope abundance of the elements.

In other words, the average chemical formula and isotope ratio of elements matter. Meanwhile, molar mass is a bulk property, reflecting the average mass of particles in a material. Second, molecular mass describes the mass of a single molecule or type of molecule. On the other hand, the unit for molar mass is grams per mole (g/mol) or kilograms per mole (kg/mol). But, they are not exactly the same as each other.įirst, molecular mass is either unitless or else reported in daltons (Da) or atomic mass units (amu or u). Most of the time, people use the terms “molar mass” and “molecular mass” interchangeably. Atomic mass of C = 12.01 atomic mass of O = 16.00.Atomic mass of Na = 22.99 atomic mass of Cl = 35.45.Example #2: Find the Molar Mass of NaClĪpply the same step and find the molar mass of table salt or NaCl. The molar mass of O 2 is 16.00 x 2 = 32.00 g/mol. Next, multiply this value by 2 (the subscript following the symbol for oxygen, O). First, look up the atomic mass (atomic weight) of the element, which is 16.00. Remember, molar mass is an average mass per mole.Īs another example, find the mass of one mole of oxygen gas. This is because the average isotope abundance of sodium in the Earth’s crust includes other isotopes besides sodium-22. Now, you know the atomic number of sodium is 11, so you may wonder why the molar mass is not exactly 22 (11 protons and 11 neutrons). The relative atomic mass is the same as the molar mass (except molar mass is in g/mol). Do this by looking up sodium (Na) on the periodic table. If there is no subscript, it’s the same as multiplying by ‘1’.Įxample #1: Find the Molar Mass of an Elementįor example, find the mass of one mole of sodium. For each element, multiply the atomic mass by the subscript following its symbol. Add up the atomic mass values of each element, according to the chemical formula.

(Note: Use different values if you are working with a known isotope.)
#Molar mass nacl how to
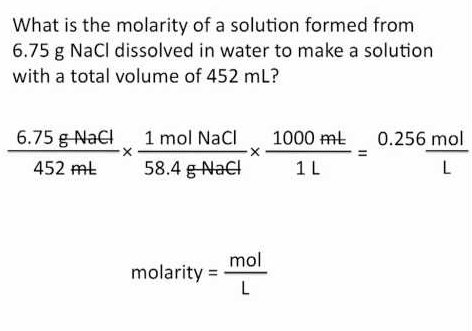
How to Find Molar Massįollow these simple steps to find the molar mass of a compound: Molar mass is an intensive property of matter, meaning its value does not depend on sample size. In chemistry, the molar mass is the mass in grams per mole (g/mol) or kilograms per mole (kg/mol) of a substance. Find it by adding up the element atomic masses. Molar mass is the mass in grams per mole of a substance. This entry was posted on Augby Anne Helmenstine (updated on August 19, 2022)


 0 kommentar(er)
0 kommentar(er)
